

Telescoped Synthesis of γ-Bromo-β-Lactones from Allylic Bromides Employing Carbon Dioxide. Vellalath, S.; Romo, D. (Special Issue dedicated to Profs. Stuart L. Schreiber and K. C. Nicolaou on their receipt of the Wolf Prize).
Impact factor : 2.42.

PUBLICATIONS
The Emerging Utility of α,β-Unsaturated Acylammonium Salts for Asymmetric Organocatalysis Vellalath, S.; Romo, D.
Impact factor : 11.26.

Utility and NMR Studies of α,β-Unsaturated Acylammonium Salts: Synthesis of Polycyclic Dihydropyranones and a Dihydropyridone. Vellalath, S.; Van, K. N.; Romo, D.
Impact factor : 2.37.

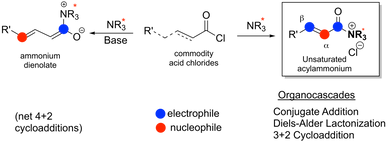
Direct Catalytic Asymmetric Synthesis of N-Heterocycles from Commodity Acid Chlorides by Employing a,b-Unsaturated Acylammonium Salts. Vellalath, S, Van, K. N.; Romo, D.
Impact factor : 11.26.

The Catalytic Asymmetric Acetalization. Kim, J. H.; Coric, I.; Vellalath, S.; List, B.
Impact factor : 11.26.
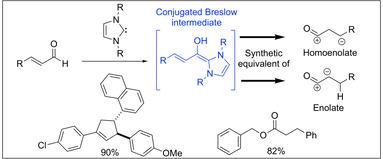
Employing homoenolates generated by NHC catalysis in carbon-carbon bond-forming reactions: state of the art. Nair, V.; Menon, R. S.; Biju, A. T.; Sinu, C. R.; Paul, R. P.; Jose, A.;
Vellalath, S.
Impact factor : 33.38

N-Phosphinyl Phosphoramide - A Chiral Brønsted Acid Motif for the Direct Asymmetric N,OAcetalization of Aldehydes. Vellalath, S.; Coric, I.; List, B.
Impact factor : 11.26

NHC-catalysed Annulation of Enals to tethered Dienones: Efficient Synthesis of Bicyclic Dienes. Nair, V.; Vellalath, S.; Babu, B. P.; Varghese, V.; Paul, R. R.; Suresh, E.
Impact factor : 3.56

Catalytic Asymmetric Transacetalization. Coric, I.; Vellalath, S.; List, B.
Impact factor : 12.11
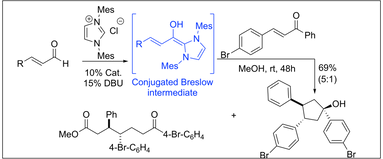
Nucleophilic Heterocyclic Carbene Catalyzed Annulation of Enals to Chalcones in Methanol: A Stereoselective Synthesis of Highly Functionalized Cyclopentanes. Nair, V.; Babu, B. P.;
Vellalath, S.; Varghese, V.; Raveendran, A. E.; Suresh, E.
Impact factor : 6.36

Direct Catalytic Asymmetric Synthesis of Cyclic Aminals from Aldehydes. Cheng, X.; Vellalath, S.; Goddard, R.; List, B.
Impact factor : 12.11

Recent Advances in Carbon-Carbon Bond-Forming Reactions Involving Homoenolates Generated by NHC catalysis. Nair, V.; Vellalath, S.; Babu, B. P.
Impact factor : 33.38
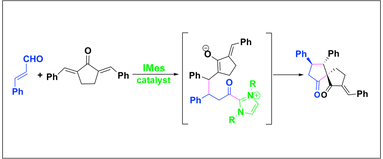
Stereoselective Synthesis of Spirocyclopentanones via N-heterocyclic Carbene-Catalyzed Reactions of Enals and Dienones. Nair, V.; Babu, B. P.; Vellalath, S.; Suresh, E.
Impact factor : 6.83
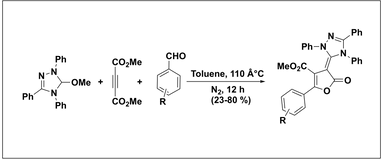
An Efficient Three-Component Reaction Involving Triazolylidene Carbene, DMAD, and Aldehydes for the Synthesis of Furanone Derivatives. Nair, V.; Mathew, S. C.; Vellalath, S.; Pillai, A. N.; Suresh, E.
Impact factor : 2.68
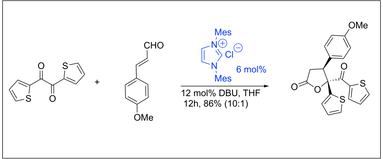
N-Heterocyclic Carbene Catalyzed Reaction of Enals and Acyclic-1,2-diones via Homoenolate: Synthesis of 4,5,5-Trisubstituted g-Butyrolactones. Nair, V.; Vellalath, S.; Poonoth, M.; Suresh, E.; Viji, S.
Impact factor : 2.68

N-Heterocyclic Carbene Catalyzed Reaction of Chalcones and Enals via Homoenolate: an Efficient Synthesis of 1,3,4-Trisubstituted Cyclopentenes. Nair, V.; Vellalath, S.; Poonoth, M.; Suresh, E.
Impact factor : 12.11
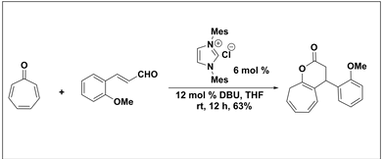
N-Heterocyclic Carbene Catalyzed [8+3] Annulation of Tropone via Homoenolate. Nair, V.; Poonoth, M.; Vellalath, S.; Suresh, E.; Thirumalai, R.
Impact factor : 4.72

Asymmetric Synthesis of Quinine: A Landmark in Organic Synthesis. Nair, V.; Menon, R. S. ; Vellalath, S. Natural Product Communications 2006, 1, 899-905 (review).
Impact factor : 0.92

Reaction of Dimethoxycarbene-DMAD Zwitterion with 1,2-Diones and Anhydrides: A Novel Synthesis of Highly Substituted Dihydrofurans and Spirodihydrofurans Nair, V.; Deepthi, A.; Poonoth, M.; Santhamma, B.; Vellalath, S.; Babu, B. P.; Mohan, R.; Suresh, E.
Impact factor : 4.72
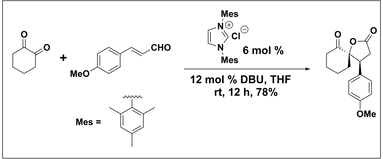
N-Heterocyclic Carbene Catalyzed Reaction of Enals and 1,2-Dicarbonyl Compounds: Stereoselective Synthesis of Spiro -Butyrolactones. Nair, V.; Vellalath, S.; Poonoth, M.; Mohan, R.; Suresh, E.
Impact factor : 6.36
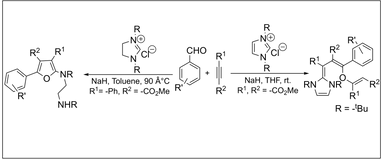
Two Unprecedented Multicomponent Reactions Involving N-Heterocyclic Carbenes, Activated Acetylenes and Aldehydes. Nair, V.; Sreekumar, V. ; Bindu, S.; Suresh, E.
Impact factor : 6.36
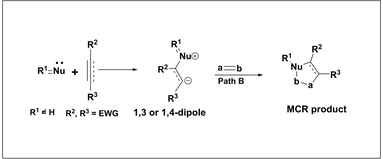
Multicomponent Reactions Based on Nucleophilic Carbenes and their Applications in Organic Synthesis. Nair, V.; Menon, R. S. ; Sreekumar, V .
Impact factor : 3.38

The Multicomponent Reaction of Dimethoxycarbene, Dimethyl Butynedioate and Electrophilic Styrenes: An Unprecedented Synthesis of Highly Substituted Cyclopentanone Acetals. Nair, V.; Beneesh, P. B.; Sreekumar, V.; Bindu, S.; Menon, R. S.; Deepthi, A.
Impact factor : 2.37

N-Heterocyclic Carbenes: Reagents, Not Just Ligands!. Nair, V.; Bindu, S.; Sreekumar
Impact factor : 11.26

A Novel Multicomponent Reaction Involving Isocyanide, Dimethyl Acetylenedicarboxylate (DMAD), and Electrophilic Styrenes: Facile Synthesis of Highly Substituted Cyclopentadienes. Nair, V.; Menon, R. S.; Beneesh, P. B.; Sreekumar, V.; Bindu, S.
Impact factor : 6.36

Unprecedented Reactivity of N-Heterocyclic Carbenes toward DMAD and Aldehydes Leading to Novel Multicomponent Reactions. Nair, V.; Bindu, S.; Sreekumar, V.; Rath, N. P.
Impact factor : 6.36

Novel Dipolar Cycloaddition Reactions of Zwitterionic Species Generated from Dimethoxycarbene and Dimethyl Acetylenedicarboxylate with Carbonyl Compounds: Facile Synthesis of Dihydrofuran Derivatives Nair, V.; Bindu, S.; Sreekumar, V.; Balagopal, L.
Impact factor : 2.68

A Novel Approach to the Synthesis of Bicyclic Lactones via an Interrupted Nazarov Reaction of gem-divinyl Dihydrofurans. Nair, V.; Bindu, S.; Sreekumar, V.; Chiaroni, A.
Impact factor : 6.36

Natural Bentonite Clay/Dilute HNO3 (40%) - A Mild, Efficient and Reusable Catalyst/Reagent System for selective mono Nitration and Benzylic Oxidations.
Impact factor : 0.92

Enantioselective Synthesis of Medium‐Sized Lactams via Chiral α,β‐Unsaturated Acylammonium Salts.
Kang, G.; Yamagami, M.; Vellalath, S.; Romo, D
Impact factor : 11.26.

(−)-Homosalinosporamide A and Its Mode of Proteasome Inhibition: An X-ray Crystallographic Study.
Groll, M.; Nguyen, H.; Vellalath, S.; Romo, D
Impact factor : 4.37.
